Solutions
Products
-

Primary mobile crushing plant
-

Independent operating combined mobile crushing station
-

Mobile secondary crushing plant
-

Fine crushing and screening mobile station
-

Fine crushing & washing mobile station
-

Three combinations mobile crushing plant
-

Four combinations mobile crushing plant
-

HGT gyratory crusher
-

C6X series jaw crusher
-

JC series jaw crusher
-

Jaw crusher
-

HJ series jaw crusher
-

CI5X series impact crusher
-

Primary impact crusher
-

Secondary impact crusher
-

Impact crusher
-

HPT series hydraulic cone crusher
-

HST hydraulic cone crusher
-
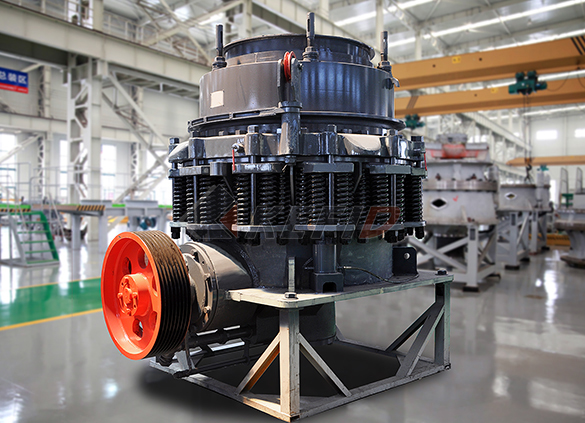
CS cone crusher
-

VSI6S vertical shaft impact crusher
-
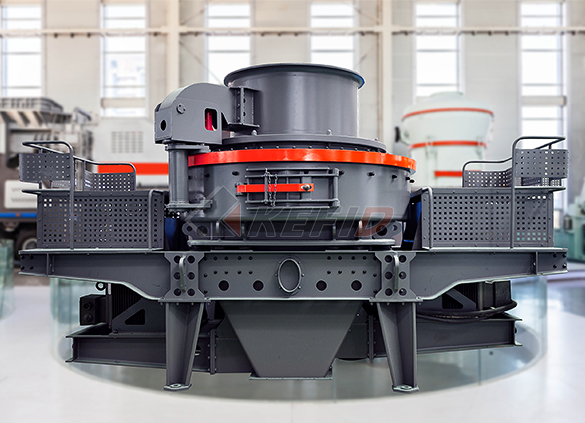
Deep rotor vsi crusher
-
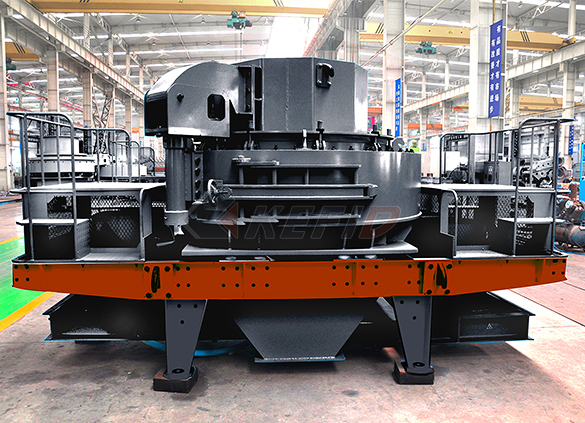
B series vsi crusher
-

Vertical grinding mill
-

Ultra fine vertical grinding mill
-

MTW european grinding mill
-

MB5X158 pendulum suspension grinding mill
-

Trapezium mill
-

T130X super-fine grinding mill
-

Micro powder mill
-

European hammer mill
-

Raymond mill
-
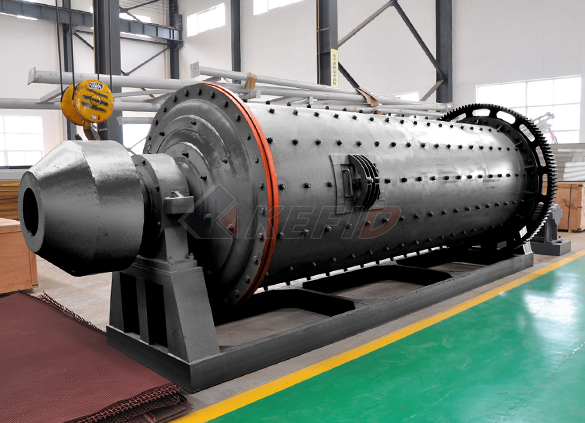
Ball mill
-
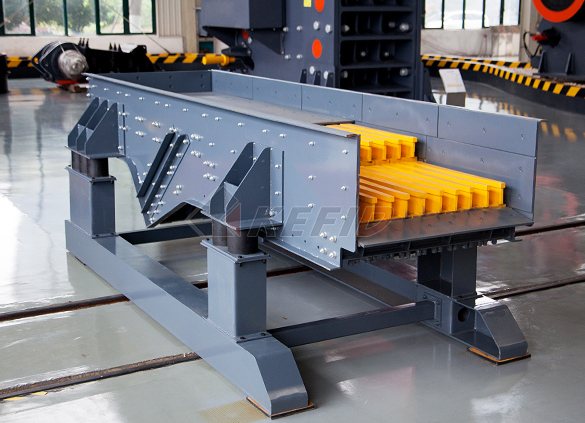
GF series feeder
-

FH heavy vibrating feeder
-

TSW series vibrating feeder
-

Vibrating feeder
-

Vibrating screen
-

S5X vibrating screen
-

Belt conveyor
-

Wheel sand washing machine
-

Screw sand washing machine
-

Rod mill
-

Dryer
-

Rotary kiln
-

Wet magnetic separator
-

High gradient magnetic separator
-

Dry magnetic separator
-

Flotation machine
-

Electromagnetic vibrating feeder
-
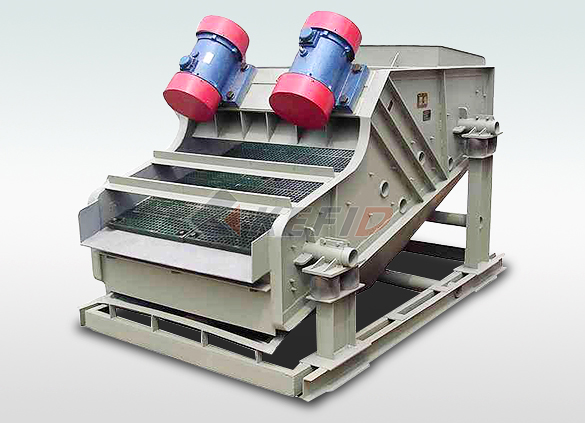
High frequency screen

Write the chemical reactions involved in the
answered Oct 31, 2018 by ShrutiBharti (344k points) selected Oct 31, 2018 by Vikash Kumar Best answer Chemical reactions involved in the process of extraction of Gold : Dilute NaCN is used for leaching the metal forming an aurocyanide complex Zn acts as a Smelting, which results in nearly pure gold, involves melting the negative terminals in a furnace at about 2,100 degrees F (1,149 degrees C) When workers add a chemical mixture known as flux to the molten material, the gold separates from the metal used to make the terminals Workers pour off the flux and then the goldExtracting Gold HowStuffWorksExtraction of gold involves leaching the metal with Oxidation reaction: The metal is recovered by diSQPlacement method : Zinc acts as a reducing agentWrite and explain the reactions involved in the

Describe the role of (i) NaCN in the extraction of
Best answer (i) Role of NaCN in the extraction of gold is to do the leaching of gold ore in the presence of air from which the gold is obtained later by replacement (ii) SiO 2 is added to copper matte to convert the leftout FeS, FeO into slagThe roasted ore of gold is leached with a solution of sodium cyanide in the presence of oxygen for many days The role of NaCN in this process is to dissolve the gold to form an aurocyanide complex, from which the metal is obtained by displacement4Au + 8NaCN + 2H2O + O2 → 4Na[Au(CN)2] + 4KOH2Na[Au(CN)2] + Zn → Na2[Zn(CN)4] + 2AuWrite the chemical reactions involved in the Silver and gold are extracted by the cyanide process (Mac Arthur Forrest process) After the preliminary crushing and concentration by froth floatation process, the ore (crushed auriferous rocks in the case of gold) is leached with dilute (04 7%) solution of sodium cyanide made alkaline by adding lime kept agitated by a current of airIn the cyanide process for extraction of gold and

What Is Aqua Regia? How Does It Dissolve Gold?
26032018 Aqua regia is an integral part of the extraction and purification processes of gold and platinum Aqua regia and its World War 2 story Back in 1940, when Hitlerled Germany invaded Denmark, Hungarian chemist George de Hevesy dissolved the Nobel Prize gold Extraction Introduction Figure 1 shows the four streams involved in the extraction process with the common nomenclature in the case when component B is separated from the mixture of A and B by means problems that are usually treated using empiric equations Main factors to be considered forPractica in Process Engineering II ExtractionThe Chemistry of the Extraction of Gold MJ Nicol, CA Fleming and RL Paul 1S1 General Principles 1511 The chemistry of gold compounds Gold is the most noble of all the metals and this is the key to both its eternal romance and its many practical uses in industry It is the only metal, forThe Chemistry of the Extraction of Gold SAIMM

Simple method for extracting gold from electrical and
gold from a leaching solution using a solvent extraction method (10) In the process presented by Biong et al, over 999% of gold was extracted from a leaching solution using solvent (Dibutyl carbinol) extraction method Park and Fray indicated that using solvent extraction method (toluene as extraction solution and tetraoctyl ammoniumAqua regia is an integral part of the extraction and purification processes of gold and platinum Aqua regia and its World War 2 story Back in 1940, when Hitlerled Germany invaded Denmark, Hungarian chemist George de Hevesy dissolved the Nobel Prize gold medals of Max von Laue and James Franck in aqua regia What Is Aqua Regia? How Does It Dissolve Gold?Silver and gold are extracted by the cyanide process (Mac Arthur Forrest process) After the preliminary crushing and concentration by froth floatation process, the ore (crushed auriferous rocks in the case of gold) is leached with dilute (04 7%) solution of sodium cyanide made alkaline by adding lime kept agitated by a current of airIn the cyanide process for extraction of gold

Extraction of Metals Methods of Extraction of
Isolation of elements in Chemistry class 12 aims to teach the students about various processes of extraction of metals from ores Very few metals such as the noble metals, ie, Gold, Silver, and Platinum etc are present in their original metallic forms in natureGold don't affect the environment; but the the mercury or sodium (potassium) cyanide used for gold extraction are dangerous materials Is equation a noun? Yes, the word 'equation' is a noun, a Word equation for gold extraction? AnswersClick here👆to get an answer to your question ️ Write the chemical reactions involved in the process of extraction of Gold Explain the role of dilute NaCN and Zn in this processWrite the chemical reactions involved in the

Gold Extraction Recovery Processes
Metallurgical ContentProcess DevelopmentSelecting a ProcessGold RecoveryAmalgamationFlotationLeachingCyanidation Process Development Considering the different gold Write the chemical reaction involved in the extraction of gold by cyanide process Also give the role of zinc in the extractionWrite the chemical reaction involved in the Extraction Introduction Figure 1 shows the four streams involved in the extraction process with the common nomenclature in the case when component B is separated from the mixture of A and B by means problems that are usually treated using empiric equations Main factors to be considered forPractica in Process Engineering II Extraction

Extraction of Gold
In precipitating the gold by zinc, the proportion required is about seven times that indicated by the equation Zn+2Au • =2Au + Zn ••, the discrepancy being due to solution of part of the zinc in the cyanide solution, with evolution of hydrogen Purity of the zinc is an important factor in counteracting this lossextraction of gold and related equations of reaction gold extraction process from electrum flowsheet for iron ore extraction processes of science hammer for impact extraction of iron ore dressing method of iron extraction extraction of ores and minerals gold ore dressing gold extraction from black sand mr4 gold extraction machineextraction and processing of gold platinum and Isolation of elements in Chemistry class 12 aims to teach the students about various processes of extraction of metals from ores Very few metals such as the noble metals, ie, Gold, Silver, and Platinum etc are present in their original metallic forms in natureExtraction of Metals Methods of Extraction of

separation of gold from its ore balanced equation
20 Oct 2014 Leaching gold with a cyanide solution remains the most widely used the chemical reaction that forms the basis of all gold cyanide leaching processes: The equation is well known, but the successful application of this of the ore is the key element to successful extraction of gold from its host rock Live ChatGold don't affect the environment; but the the mercury or sodium (potassium) cyanide used for gold extraction are dangerous materials Is equation a noun? Yes, the word 'equation' is a Word equation for gold extraction? AnswersMetallurgical ContentProcess DevelopmentSelecting a ProcessGold RecoveryAmalgamationFlotationLeachingCyanidation Process Development Considering the different gold Gold Extraction Recovery Processes

Write the chemical reactions involved in the
Write the chemical reactions involved in the extraction of gold by the cyanide process Also, give the role of zinc in the extractionWrite the chemical reaction involved in the extraction of gold ? Ask for details ; Follow Report by Shashiverma12 26062018 Log in to add a commentWrite the chemical reaction involved in the Extraction Introduction Figure 1 shows the four streams involved in the extraction process with the common nomenclature in the case when component B is separated from the mixture of A and B by means problems that are usually treated using empiric equations Main factors to be considered forPractica in Process Engineering II Extraction

1 Write equations for the following processes,
1 Write equations for the following processes, involved in the extraction of the elements from their ores: (a) the reduction of boron oxide by Mg; (b) the result of the addition of hot aqueous NaOH to a mixture of solid Al2O3 and Fe2O3; (c) the reaction of CO2 with aqueous Na[Al(OH)4]